News.hust.edu.cn (Correspondent: Zhengjun Chen) On December 4th, Nature Communications published the new results of the research on the solid-liquid interface of humid ionic liquid by Prof. Guang Feng's team in the State Key Laboratory of Coal Combustion and the School of Energy and Power Engineering. The paper has a title of "Minimizing the electrosorption of water from humid ionic liquids on electrodes", with HUST as the primary affiliation.
The ionic liquid consists only of cations and anions, remaining in liquid state at room temperature. In addition to the advantages of good thermal stability, low volatility, non-flammability and non-explosion, ionic liquid has a higher working voltage (4~6 V) than the commonly used aqueous or organic electrolytes. In recent years, as an electrolyte used in energy storage devices (such as batteries and supercapacitors), electrowetting technology, field-effect transistors, electrochemical sensors, etc., ionic liquid has been receiving more and more concerns and attention. However, due to its intrinsic feature of hygroscopicity, water in the ionic liquid is always difficult and even impossible to be completely removed. Studying the influence of water content on ionic liquids, Prof. Feng’s previous work (ACS Nano 2014, 8, 11685) predicted by naoscale simulation that in the forming process of the electrical double layer of the interface between the electrode and ion liquid, the water in ionic liquid would be enriched on the polarized electrode surface, which would potentially make the operating voltage of device/equipment become smaller, and ultimately result in reducing the working performance. Subsequently, this theoretical prediction is verified by many experiments and simulations. Therefore, how to reduce the negative influence of water adsorption from the humid ionic liquid on the electrode surface has become one of the crucial challenges that need urgently to be solved in the field of ionic liquid electrolyte applications.
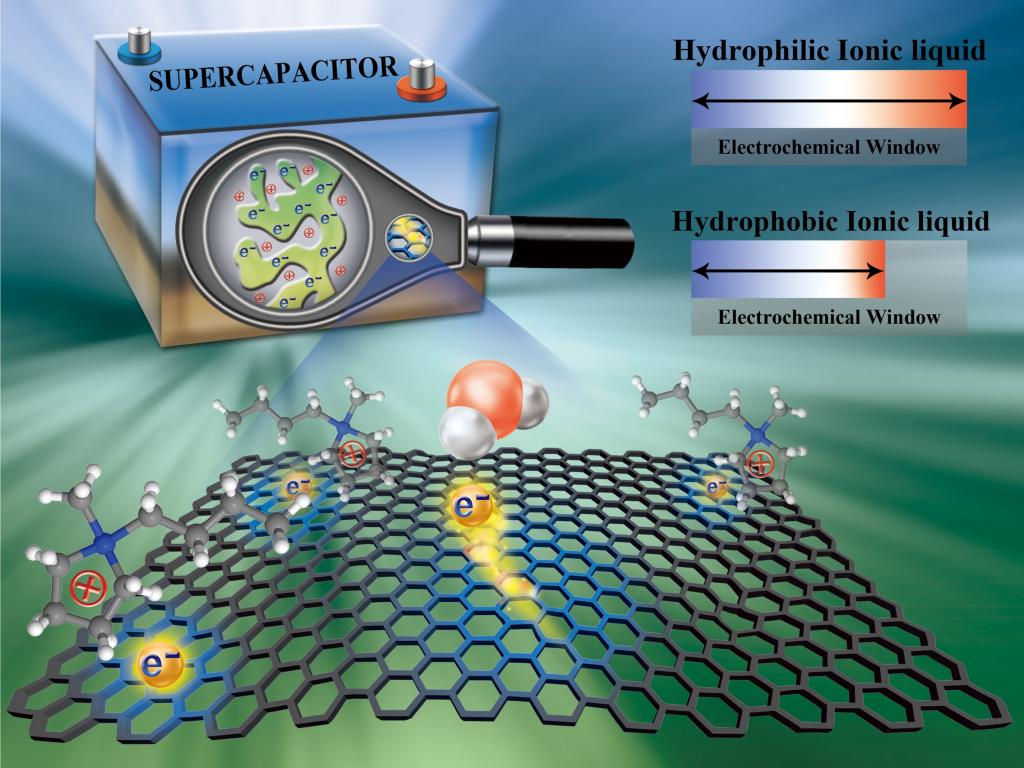
Prof. Feng’s team, adopting molecular dynamics simulation and taking the hydrophilicity/hydrophobicity of ionic liquid as a focus, found that the utilization of hydrophilic ionic liquid can effectively prevent water in humid ionic liquid from adsorbing on the surface of negatively charged electrodes, and thus avoid the reduction of operating voltage. This solution is suitable for electrodes with different materials. According to the analysis of the simulation results, the corresponding microscopic mechanisms and realization principles were further elaborated: van der Waals and electrostatic interactions from electrodes and ionic liquids determine where the water should be, making the water in the hydrophilic ionic liquid away from electrode surface. The results of the molecular simulation were verified experimentally by their collaborators from Xiamen University.
From the perspective of microscopic interface and energy/mass transfer, taking the electrode-electrolyte solid-liquid interface in energy storage devices as the research focus, this work revealed the working mechanism of the influence of different ionic liquids on the adsorbed water on the polarized electrode surface, and gave the solution and realization principles. The results could not only help to understand the energy storage mechanism of ionic liquid supercapacitors, but also provide new ideas and solutions for the development and design of supercapacitors, which would also be beneficial to the application of ionic liquids in other fields taking electrode-electrolyte interface as the key component. They furthermore help to utilize the simulation and modeling to investigate the micro/nanoscale interface and energy transfer phenomenon.
The first and second authors of this paper are Sheng Bi and Runxi Wang respectively, the Ph.D. candidates of the School of Energy and Power Engineering. The corresponding authors are Prof. Guang Feng, Prof. Jiawei Yan from Xiamen University, and Prof. Alexei Kornyshev from Imperial College London. This work was funded by the National Natural Science Foundation of China (51876072) and Shenzhen Basic Research Project (JCYJ20170307171511292).
Link to the Paper: https://doi.org/10.1038/s41467-018-07674-0
