Lithium-ion batteries (LIBs) are the dominating power sources for electric vehicles and arepenetrating into the large-scale energy storage systems. After 5-10 years’ service, the accumulated end-of-life LIBs are estimated up to 464,000 tons in 2025 over the world, which makes their recycling an urgent task with respect to resource and environmental consideration. Cathode active materials of spent LIBs contain valuable metals (e.g., lithium, nickel and cobalt) and various approaches have been developed for their recycling, such as the recovery of valuable metals through classical pyrometallurgy, hydrometallurgy and bio-metallurgy techniques, as well as the emerging direct regeneration. Among all the operations, separation of active material layer from Al foil of cathode becomes one of the most important procedures. The subsequent recycling of active material and Al foil can be readily promoted after their efficient separation.
Yongming Sun’ group proposed a reaction-passivation driven mechanism for efficient separation of Al foil and cathode active material layer from spent LIBs. 60 g of Al foil and 636 g of LiNi0.55Co0.15Mn0.3O2(Ni55) from a 102 Ah spent cell were facilely separated using a PA solution in 5 mins with > 99.9 % separation efficiency. An ultrathin, dense aluminum-phytic acid complex layer is in-situ formed on Al foil immediately after its contact with phytic acid, which suppresses continuous Al corrosion. Besides, the dissolution of transitional metal from Ni55 is negligible and good structural integrity of is well-maintained during the processing. This work demonstrates a feasible approach for Al foil-active material layer separation of cathode and can promote the green and energy-saving battery recycling towards practical applications.
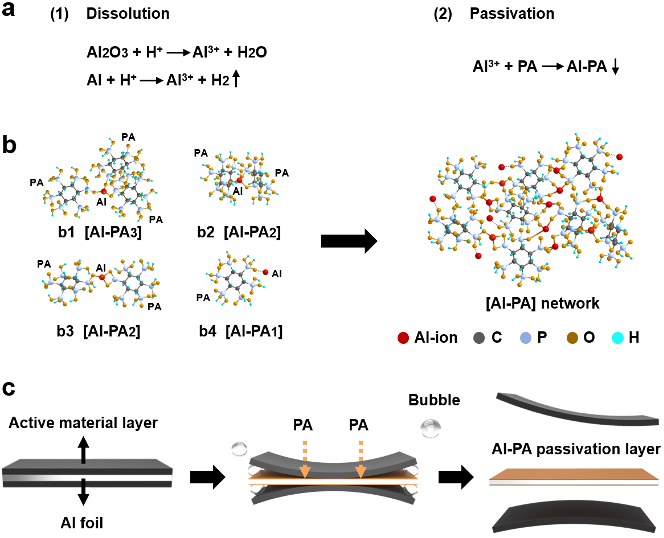
Figure 1. Schematic of reaction-passivation driven separation of Al foil and active material layer. (a) The reaction-passivation mechanism of Al foil with PA. (b) The plausible connectivity between PA and Al ions. (c) Schematic of the separation process between active material and Al foil in PA solution.

Figure 2. Separation of Al foil and active material layer of Ni55 cathode. (a) Digital images of Al foil-Ni55 layer separation process in PA solution. (b) 11.5 m-length cathode of a 102 Ah spent cell. The inset in Figure b showed the digital image of a 102 Ah cell for Al foil-Ni55 layer separation. (c) 11.5 m-length Al foil and (d) Ni55 layer after their separation. (e) Al, Li, Ni, Co and Mn contents in PA solution after Al foil-Ni55 layer separating operation. As a control, the separation experiment of Ni55 cathode was also conducted using HCl solution, and the Li, Ni, Co and Mn contents in HCl solution was measured. (f) XRD patterns of the separated Al foil and Ni55 layer.
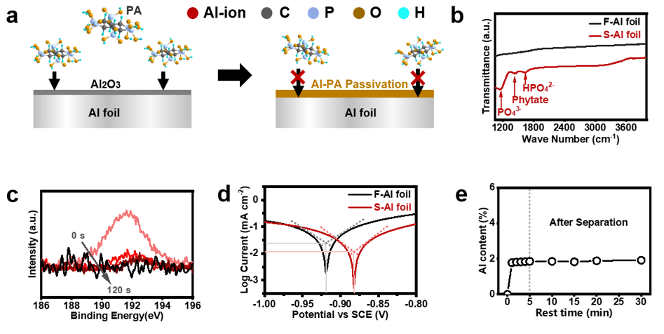
Figure 3. Characterizations of Al foil and PA solutions under different conditions. (a) Schematic of the reaction-passivation mechanism for Al foil-Ni55 layer separation. During the separation, PA reacted with the surficial Al2O3and metallic Al to produce an ultrathin, dense Al-PA layer on Al foil, which could terminate the continuous corrosion of Al foil. (b) The FT-IR spectra of F-Al and S-Al foils. (c) High-resolution P 2sXPS spectra of S-Al foil upon the Ar+sputtering. (d) Tafel curves of F-Al and S-Al foils. (e)The Al contents in PA solution caused by the dissolution of Ni55 during the Al foil-Ni55 layer separation process.

Figure 4. Economic and environmental analysis of PA-direct and other recycling approaches. (a) Brief schematic of the PA-direct. (b) Energy consumption, (c) GHG emission, (d) water consumption, (e) cost, (f) revenue and (g) profit for PA-direct, General-direct, Pyro and Hydro. (h) The overall cost of manufacturing 1 kg-Ni55 cathode from raw and recycled materials. (i) Comprehensive comparison of different recycling approaches.
Related work has been published on Nature Communications (https://doi.org/10.1038/s41467-023-40369-9) on August, 2, 2023, with the title Reaction-passivation mechanism driven materials separation for recycling of spent lithium-ion batteries. The first unit for this research is the Wuhan National Laboratory for Optoelectronics Huazhong University of Science and Technology, and it has been funded by the National Natural Science Foundation of China (No. 52072137).