Recently, Prof. Zhang Zhihong from Wuhan National Laboratory for Optoelectronics-Huazhong University of Science and Technology published a research paper entitled "Three-dimensional mapping of hepatic lymphatic vessels and transcriptome profiling of lymphatic endothelial cells in healthy and diseased livers" in the journal Theranostics. The study developed a method for the spatiotemporal sequential injection of antibodies (STSI-Ab) to selectively label hepatic LyECs in vivo, then, the 3D imaging results provided high-resolution structural mapping of hepatic lymphatic vessels(LVs)(Figure 1).

Figure1. Three-dimensional mapping of the mouse hepatic lymphatics. (A) Mouse hepatic lymphatic 3D imaging flow diagram. (B-D) Green vessels represent mouse liver lymph vessels labeled with STSI-Ab, blue vessels represent the portal vein, and red vessels represent the central vein. The three images in the bottom row are the enlarged images in the white box in the top row.
Hepatic lymphatics are essential for liver homeostasis and immune function. However, the 3D structure and spatial distribution of hepatic LVs need to be confirmed. Moreover, the molecular information of hepatic lymphatic endothelial cells (LyECs) needs to be further studied. The bottleneck is the lack of specific markers or labeling methods for hepaticLyECs. In this study, the team proposed a method for the spatiotemporal sequential injection of antibodies(STSI-Ab) to selectively label hepatic LyECs in vivo. The STSI-Ab method achieved selective labeling of the mouse hepatic lymphatic network. Three-dimensional fluorescence imaging results of the STSI-Ab mouse liver lobe clearly showed that hepatic LVs entangled with the portal vein but were not present in the central vein. The imaging data inspired a novel hepatic lobule structure model with an added set of LVs in the portal area (Figure 2). Furthermore, deep transcriptome sequencing of isolated hepatic LyECs and Masson’s trichrome staining results suggested that hepatic LyECs might be an important source of collagen fibers deposited in the portal area during the process of liver fibrosis and bile duct ligation (BDL).
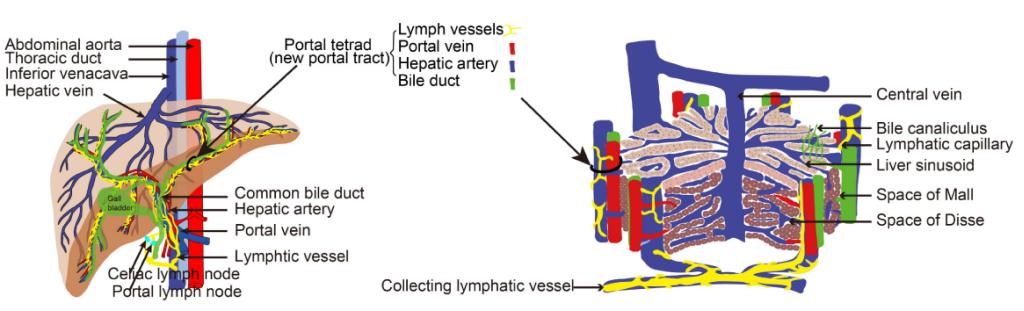
Figure2. Schematic diagram of liver vessels and hepatic lobule with portal tetrad.
In conclusion, we proposed an STSI-Ab method for selectively labelinghepaticLVs, distinguishing thehepaticLVs from other vessels, and mapping their 3D structure. This study revealed previously unknown intact hepatic lymphatics from lymphatic capillaries to large lymphatic vessels (LVs) with definite spatial coordinates, which provided a reference for clinical liver lymphatic angiography. Furthermore, we established a new method for accurately sorting hepatic LyECs for transcriptome sequencing. Our data suggested that hepatic LyECs may be an important source of collagen fibers deposited in the portal area during the process of liver fibrosis and BDL, which would be considered a potential target for reversing the liver fibrosis process.
This work was supported by the National Key Research and Development Program of China (2017YFA0700403 and 2017YFA0700402), the National Natural Science Foundation of China (81901691), the Fundamental Research Funds for the CentralUniversities (2019XMBZ022), the China PostdoctoralScience Foundation (2019M662637), the Program for HUST Academic Frontier Youth Team (Zhang, Z.H.), and the Innovation Fund of WNLO. Prof. Zhihong Zhang from Huazhong University of Science and Technology and Prof. Qingming Luo from Hainan University are co-corresponding authors of the paper. Dr. Songlin Huang, Borui Li, and Zheng Liu contributed equally to the paper.
Paper link: https://www.thno.org/v13p0639.htm